3.3 Component 3 – Chemistry: Elements, mixtures and compounds
Matter is composed of tiny particles called atoms and there are about 100 naturally occurring types of atoms called elements.
Elements are shown in the periodic table and are either metals or non-metals. Atoms are the building blocks for all substances. When two or more elements combine chemically a compound is produced.
Different substances have different combinations of atoms joined together in different ways, which gives them different properties, such as whether they are solid, liquid or gaseous at room temperature. Many materials we use are mixtures. Mixtures can be separated by processes such as filtration. Polymers have many useful applications.
Atoms, elements and compounds
Students should have knowledge and understanding of the following content.
| Content | Additional guidance and suggested TDAs | Specification reference GCSE Combined Science: Trilogy | Specification reference GCSE Combined Science: Synergy |
|---|---|---|---|
| Outcome 1 | |||
|
All substances are made of atoms. An atom is the smallest part of an element that can exist. |
5.1.1.1 |
||
|
A substance that is made of only one sort of atom is called an element. There are about 100 different elements. Elements are shown in the periodic table. Metals are towards the left and the bottom of the periodic table and non-metals towards the right and the top of the periodic table. Students should know that most of the elements are metals. |
5.1.1.1 and 5.1.2.3 |
4.5.1.1 and 4.5.1.2 |
|
|
Elements in the same group of the periodic table have similar chemical properties. |
Knowledge of groups is limited to Group 1 as reactive metals and Group 7 as reactive non-metals. |
5.1.2.1 |
4.5.1.1 |
| Outcome 2 | |||
|
When elements react, their atoms join with other atoms to form compounds. |
5.1.1.1 |
4.5.2.1 |
|
|
Some compounds are made from metals combined with non-metals, for example sodium chloride and magnesium oxide. Students should be able to recognise simple compounds from their names, eg sodium chloride, magnesium oxide, carbon dioxide. Some compounds are made from only non-metals, for example carbon dioxide. |
5.2.1.2 |
4.6.2.1 |
|
|
Chemical reactions can be represented by word equations. Students should be able to write word equations for reactions of metals and non-metals, reactions of non-metals to produce oxides, and the other chemical reactions in this specification. |
5.1.1.1 |
4.5.2.1 |
How structure affects properties
Students should have knowledge and understanding of the following content.
| Content | Additional guidance and suggested TDAs | Specification reference GCSE Combined Science: Trilogy | Specification reference GCSE Combined Science: Synergy |
|---|---|---|---|
| Outcome 3 | |||
|
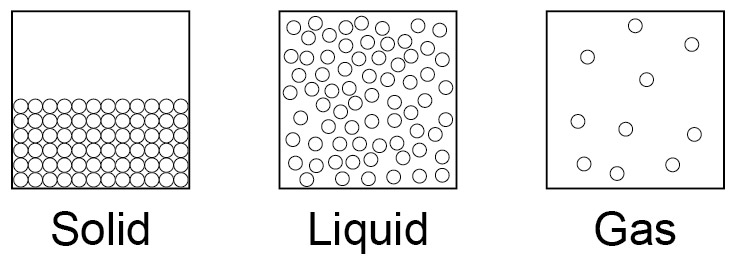
The three states of matter are solid, liquid and gas. Melting and freezing take place at the melting point, boiling and condensing take place at the boiling point. The three states of matter can be represented by a simple model. In this model, particles are represented by small solid spheres.
|
Students should be able to use models of particles as small spheres to represent the three states of matter. |
5.2.2.1 |
4.1.1.1 |
|
When a solid melts to become a liquid the particles are able to move about but stay close together. When a liquid boils and becomes a gas the particles separate and move about rapidly. Substances with high melting points have strong forces that hold their particles together. Substances with low boiling points have weak forces between their particles. |
Suggested activity for TDA Compare the melting points of a range different substances, eg candle wax, beeswax polish, butter, margarine, cooking fat. |
5.2.2.1 |
4.6.2.5 |
| Outcome 4 | |||
|
Diamond and graphite are forms of the element carbon with different properties because of their different structures. Diamond is hard because the carbon atoms are joined together in a giant three-dimensional structure. Graphite is slippery because the carbon atoms are joined together in layers that can slide over each other. |
Students should be able to recognise diamond and graphite from diagrams of their structures. |
5.2.3.1 and 5.2.3.2 |
4.8.1.1 |
Separating mixtures
Students should have knowledge and understanding of the following content.
| Content | Additional guidance and suggested TDAs | Specification reference GCSE Combined Science: Trilogy | Specification reference GCSE Combined Science: Synergy |
|---|---|---|---|
| Outcome 5 | |||
|
A mixture contains two or more substances not chemically combined together. Mixtures can be separated by processes such as filtration, distillation, crystallisation and chromatography. |
Students should be able to select methods from those given to separate simple mixtures. Suggested activity for TDA Compare the time needed to filter mixtures of water and calcium carbonate that has different particle sizes. |
5.1.1.2 |
4.2.2.4 |
| Outcome 6 | |||
|
Paper chromatography can be used to separate mixtures and can give information to help identify substances. In paper chromatography a solvent moves through the paper carrying different compounds different distances. |
Suggested activity for TDA Investigate the different colours in inks or food colours using paper chromatography. |
5.8.1.3 |
4.2.2.4 |
Metals and alloys
Students should have knowledge and understanding of the following content.
| Content | Additional guidance and suggested TDAs | Specification reference GCSE Combined Science: Trilogy | Specification reference GCSE Combined Science: Synergy |
|---|---|---|---|
| Outcome 7 | |||
|
Unreactive metals, such as gold, are found in the Earth as the metal itself, but most metals are found as compounds that require chemical reactions to extract the metal.
Metals less reactive than carbon can be produced by heating the metal compounds in the ore with carbon. Ores contain enough metal to make it economic to extract the metal. Large amounts of rock need to be quarried or mined to get metal ores. |
5.4.1.3 |
4.8.2.1 |
|
|
We should recycle metals to save resources and limit environmental impacts. Students should be able to describe the social, economic and environmental impacts of mining ores and recycling metals. |
5.10.2.2 |
4.8.2.9 |
|
| Outcome 8 | |||
|
Metals have giant structures of atoms with strong bonds between the atoms and so most metals have high melting points. |
Suggested activity for TDA Compare the properties, such as conductivity or density, of some metals. |
5.2.2.7 |
4.6.2.7 |
|
Metals are good conductors of electricity and thermal energy. Copper has properties that make it useful for electrical wiring and plumbing. |
The properties of copper are limited to its ability to conduct electricity easily and the ease with which it can be worked. |
5.2.2.8 |
4.6.2.7 |
|
Aluminium is a useful metal because of its low density and resistance to corrosion. |
No knowledge of the extraction process of aluminium is required. |
||
| Outcome 9 | |||
|
Most metals in everyday use are alloys. Pure iron, gold and aluminium are too soft for many uses and so are mixed with small amounts of other elements to make alloys, which are harder for everyday use. |
Knowledge of the composition of specific alloys is not required. |
5.2.2.7 |
4.6.2.7 |
| Most iron is converted into steels. Steels are alloys since they are mixtures of iron with carbon and other metals. | Suggested activity for TDA Investigate the hardness of different alloys or steels. |
Polymers
Students should have knowledge and understanding of the following content.
| Content | Additional guidance and suggested TDAs | Specification reference GCSE Combined Science: Trilogy | Specification reference GCSE Combined Science: Synergy |
|---|---|---|---|
| Outcome 10 | |||
|
Polymers such as poly(ethene), poly(propene) polystyrene and PVC are made from small compounds called monomers that join together to form very long chains. Polymers are waterproof, resistant to chemicals, and can be moulded, so they have many useful applications as packaging materials, pipes and containers. |
Common names of these polymers will be accepted. Knowledge of the names of other polymers is not required. |
5.2.2.5 |
4.6.2.4 |
| Many polymers are not biodegradable, so they are not broken down by microbes. This can lead to problems with waste disposal. | Suggested activity for TDA Compare the biodegradability of different polymers and other materials. |