Movement can take place over small and large distances, and can happen at very different speeds. Crashes, bangs and collisions can take place between sub-atomic particles or between large, moving vehicles. Scientists try to learn as much as possible about these interactions so that they can make it safer and healthier to travel and keep our homes and cities running.
4.7.1 Forces and motion
Forces can change the motion of objects. Objects can move in a straight line at a constant speed. They can also change their speed and/or direction (accelerate or decelerate). This topic shows how observations of moving objects can be accounted for in terms of Newton’s laws of motion. Graphs can help to describe the movement of objects. These may be distance–time or velocity–time graphs. When an object speeds up or slows down, its kinetic energy increases or decreases. The forces that cause the change in speed do so by doing work. The topic ends by showing that these ideas can be applied to the issue of road safety.
The required practical in this topic is an investigation of the effects of varying the force and/or mass on the acceleration of an object.
In this topic students have to be able to apply formulae relating distance, time and speed, for uniform motion and for motion with uniform acceleration, and calculate average speed for non-uniform motion (MS 1a, 1c, 2b, 3c).
4.7.1.1 Speed and velocity
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain the vector–scalar distinction as it applies to displacement, distance, velocity and speed. | Distance is how far an object moves. Distance does not involve direction. Distance is a scalar quantity. Displacement includes both the distance an object moves, measured in a straight line from the start point to the finish point, and the direction of that straight line. Displacement is a vector quantity. Speed does not involve direction. Speed is a scalar quantity. The velocity of an object is its speed in a given direction. Velocity is a vector quantity. | |
Recall typical speeds encountered in everyday experience for wind and sound, and for walking, running, cycling and other transportation systems. | The speed of a moving object is rarely constant. When people walk, run or travel in a car their speed is constantly changing. The speed that a person can walk, run or cycle depends on many factors, including age, terrain, fitness and distance travelled. Typical mean values are: - walking 1.5 m/s
- running 3 m/s
- cycling 6 m/s.
It is not only moving objects that have varying speed. The speed of sound and the speed of the wind also vary. A typical value for the speed of sound is 330 m/s. | |
4.7.1.2 Distance, speed and time
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Make measurements of distances and times, calculate speeds, and make and use graphs of these to determine the speeds and accelerations involved. | The distance travelled by an object moving at constant speed increases with time. distance, s , in metres, m speed, v , in metres per second, m/s time, t , in seconds, s If an object moves along a straight line, how far it is from a certain point can be represented by a distance–time graph. The speed of an object can be calculated from the gradient of its distance–time graph. (HT only) If an object is accelerating, its speed at any particular time can be determined by drawing a tangent and measuring the gradient of the distance–time graph at that time. | MS 3b, 3c Recall and apply this equation. WS 4.5, MS 1c, 3b, 3c Use ratios and proportional reasoning to convert units and to compute rates. WS 1.2, 3.5, MS 4a, 4b, 4c, 4d, 4f Relate changes and differences in motion to appropriate distance–time, and velocity–time graphs, and interpret lines, slopes and enclosed areas in such graphs. MS 1a, 1c, 2f, 3c Calculate average speed for non-uniform motion. |
4.7.1.3 Circular motion (HT only)
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain with examples that motion in a circular orbit involves constant speed but changing velocity (qualitative only). | The velocity of an object is its speed in a given direction. Velocity is a vector quantity. When an object moves in a circle the direction of the object is continually changing. This means that an object moving in a circle at constant speed has a continually changing velocity. | |
4.7.1.4 Free fall
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall the acceleration in free fall and estimate the magnitudes of everyday accelerations. | Acceleration is the rate at which the velocity of an object changes. acceleration, a , in metres per second squared, m/s2 change in velocity, ∆ v , in metres per second, m/s time, t , in seconds, s An object that slows down (decelerates) has a negative acceleration. The acceleration of an object can be calculated from the gradient of a velocity–time graph. (HT only) The distance travelled by an object (or displacement of an object) can be calculated from the area under a velocity–time graph. | WS 1.2, 3.3, MS 3b, 3c Recall and apply this equation. WS 1.2, 3.5, MS 4a, 4b, 4c, 4d, 4f, 5c Relate changes and differences in motion to appropriate velocity–time graphs, and interpret lines and slopes to determine acceleration. (HT only) Interpret enclosed areas in such graphs to determine distance travelled (or displacement). (HT only) Measure, when appropriate, the area under a velocity–time graph by counting squares. |
| | The following equation applies to uniform motion: final velocity, v , in metres per second, m/s initial velocity, u , in metres per second, m/s acceleration, a , in metres per second squared, m/s2 distance, s , in metres, m Near the Earth’s surface any object falling freely under gravity has an acceleration of about 9.8 m/s2 . An object falling through a fluid initially accelerates due to the force of gravity. Eventually the resultant force will be zero and the object will move at its terminal velocity. | WS 1.2, 3.3, MS 3c Apply this equation, which is given on the Physics equation sheet . |
4.7.1.5 Newton’s First Law
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Apply Newton’s First Law to explain the motion of objects moving with uniform velocity and also objects where the speed and/or direction change. | Newton’s First Law: If the resultant force acting on an object is zero and: - the object is stationary, the object remains stationary
- the object is moving, the object continues to move at the same speed and in the same direction. So the object continues to move at the same velocity.
| |
4.7.1.6 Newton’s Second Law
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Apply Newton’s Second Law in calculations relating forces, masses and accelerations. | Newton’s Second Law: The acceleration of an object is proportional to the resultant force acting on the object, and inversely proportional to the mass of the object. force, F , in newtons, N mass, m , in kilograms, kg acceleration, a , in metres per second squared, m/s2 | MS 3a Recognise and be able to use the symbol: - for proportionality, α
- that indicates an approximate value or answer, ̴
WS 1.2, 3.3, MS 3c Recall and apply this equation. |
(HT only) Explain that inertial mass is a measure of how difficult it is to change the velocity of an object and that it is defined as the ratio of force over acceleration. | (HT only) The tendency of objects to continue in their state of rest or of uniform motion is called inertia. Inertial mass is a measure of how difficult it is to change the velocity of an object. Inertial mass is defined by the ratio of force over acceleration. | |
Required practical activity 14: investigate the effect of varying the force on the acceleration of an object of constant mass and the effect of varying the mass of an object on the acceleration produced by a constant force.
AT skills covered by this practical activity: physics AT 1, 2 and 3.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.1.7 Newton’s Third Law
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall Newton’s Third Law and apply it to examples of equilibrium situations. | Newton’s Third Law: Whenever two objects interact, the forces they exert on each other are equal and opposite. | |
4.7.1.8 Momentum (HT only)
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Define momentum and describe examples of momentum in collisions. | Momentum is a property of moving objects. Momentum is defined by the equation: momentum, p , in kilograms metre per second, kg m/s mass, m , in kilograms, kg velocity, v , in metres per second, m/s In a closed system, the total momentum before an event is equal to the total momentum after the event. This is called conservation of momentum. | WS 1.2, 3.3, MS 3c Recall and apply this equation. WS 1.2 Use the concept of momentum as a model to analyse an event such as a collision. |
4.7.1.9 Kinetic energy
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Calculate the amounts of energy associated with a moving body. Describe all the changes involved in the way energy is stored when a system changes for common situations: a moving object hitting an obstacle, or an object being accelerated by a constant force. | The kinetic energy of a moving object depends on the mass and the velocity of the object. kinetic energy, E k , in joules, J mass, m , in kilograms, kg speed, v , in metres per second, m/s | WS 1.2, 3.3, MS 3c Recall and apply this equation. |
4.7.1.10 Stopping distances
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain the factors which affect the distance required for road transport vehicles to come to rest in emergencies and the implications for safety. | The stopping distance of a vehicle is the sum of the distance the vehicle travels during the driver’s reaction time (thinking distance) and the distance it travels under the braking force (braking distance). For a given braking force the greater the speed of the vehicle, the greater the stopping distance. The braking distance of a vehicle can be affected by wet or icy weather and poor condition of the vehicle’s brakes or tyres. A driver’s reaction time (see The human nervous system ) can be affected by tiredness, drugs and alcohol. Distractions may also affect a driver’s ability to react. | WS 3.6 Analyse a given situation to explain why braking could be affected. WS 1.5 Discuss the implications for safety. WS 3.5, MS 4a Interpret graphs relating speed to stopping distance for different types of vehicles. WS 1.5, 2.2, MS 1a, 1c Evaluate the effect of various factors on thinking distance based on given data. |
Explain the dangers caused by large decelerations. Describe all the changes involved in the way energy is stored when a system changes, for common situations, like a vehicle slowing down. | When a force is applied to the brakes of a vehicle, work done by the friction force between the brakes and the wheel reduces the kinetic energy of the vehicle and the temperature of the brakes increases. The greater the speed of a vehicle the greater the braking force needed to stop the vehicle in a certain distance. The greater the braking force the greater the deceleration of the vehicle. Large decelerations may lead to brakes overheating and/or loss of control. | WS 1.5, MS 1d (HT only) Estimate the forces involved in typical situations on a public road. |
4.7.2 Electricity
A quantitative study of electric currents in circuits leads on to an introduction of the alternating currents used in the mains electricity supply. Electricity is distributed to consumers via the National Grid. The rate of energy transfer depends on the power of the appliances connected to the supply.
In this topic students have to be able to apply the equations relating potential difference, current, quantity of charge, resistance, power, energy and time, and solve problems for circuits which include resistors in series, using the concept of equivalent resistance (MS 1c, 3b, 3c, 3d).
There are two required practicals in this topic. One is to investigate the I–V characteristics of a variety of circuit elements at constant temperature. The other is to set up and use appropriate circuits to investigate factors that affect the resistance of an electrical component. The practical activities give students experience of the apparatus and techniques related to the use of circuit diagrams to construct and check series and parallel circuits that include a variety of common circuit components.
4.7.2.1 Electric current
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that current is a rate of flow of charge, that for a charge to flow a source of potential difference and a closed circuit are needed and that a current has the same value at any point in a single closed loop. Recall and use the relationship between quantity of charge, current and time. | For electrical charge to flow through a closed circuit the circuit must include a source of potential difference. Electric current is a flow of electrical charge. The size of the electric current is the rate of flow of electrical charge. charge flow, Q , in coulombs, C current, I , in amperes, A (amp is acceptable for ampere) time, t , in seconds, s A current has the same value at any point in a single closed loop. | WS 3.3, MS 3b, 3c Recall and apply this equation. |
4.7.2.2 Current, resistance and potential difference
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that current (I) depends on both resistance (R) and potential difference (V) and the units in which these are measured; recall and apply the relationship between I, R and V, and explain that for some resistors the value of R remains constant. | The current through a component depends on both the resistance of the component and the potential difference across the component. The greater the resistance of the component the smaller the current for a given potential difference across the component. potential difference, V , in volts, V current, I , in amperes, A (amp is acceptable for ampere) resistance, R , in ohms, Ω The current through an ohmic conductor (at a constant temperature) is directly proportional to the potential difference across the conductor. This means that the resistance remains constant as the current changes. | WS 3.3, MS 3c Recall and apply this equation. |
Explain that in other types of resistor the value of R can change as the current changes; explain the design and use of circuits to explore such effects – including lamps, diodes, thermistors and light-dependent resistors (LDRs). | The resistance of components such as lamps, diodes, thermistors and LDRs is not constant; it changes with the current through the component. The resistance of a filament lamp increases as the temperature of the filament increases. The current through a diode flows in one direction only. The diode has a very high resistance in the reverse direction. The resistance of a thermistor decreases as the temperature increases. The resistance of an LDR decreases as light intensity increases. | WS 1.2, 3.5, MS 4c, 4d, 4e Use graphs to determine whether circuit components are linear or non-linear and relate the curves produced to the function and properties of the component. |
Required practical activity 15: use circuit diagrams to construct appropriate circuits to investigate the I–V characteristics of a variety of circuit elements including a filament lamp, a diode and a resistor at constant temperature.
AT skills covered by this practical activity: physics AT 6 and 7.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
Required practical activity 16: use circuit diagrams to set up an appropriate circuit to investigate the factors affecting the resistance of an electrical component. This should include:
- the length of a wire at constant temperature
- combinations of resistors in series and in parallel.
AT skills covered by this practical activity: physics AT 1, 6 and 7.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.2.3 Series and parallel circuits
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe the difference between series and parallel circuits; explain why, if two resistors are in series, the net resistance is increased, whereas with two in parallel the net resistance is decreased (qualitative explanation only). Calculate the currents, potential differences and resistances in direct current (dc) series circuits, and explain the design and use of such circuits for measurement and testing purposes. | There are two ways of joining electrical components: in series and in parallel. Some circuits include both series and parallel parts. For components connected in series: - there is the same current through each component
- the total potential difference of the power supply is shared between the components
- the total resistance of two components is the sum of the resistance of each component.
resistance, R , in ohms, Ω For components connected in parallel: - the potential difference across each component is the same
- the total current through the whole circuit is the sum of the currents through the separate components
- the total resistance of two resistors is less than the resistance of the smallest individual resistor.
| WS 3.3, MS 1c, 3b, 3c, 3d Solve problems for circuits which include resistors in series using the concept of equivalent resistance. WS 3.3, MS 1c, 3b, 3c, 3d Calculate the currents, potential differences and resistances in dc series circuits. Calculating the total resistance of two resistors joined in parallel is not required. |
4.7.2.4 Circuit elements
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Represent in dc series circuits with the conventions of positive and negative terminals, the symbols that represent common circuit elements, including diodes, LDRs and thermistors. | Circuit diagrams use standard symbols. 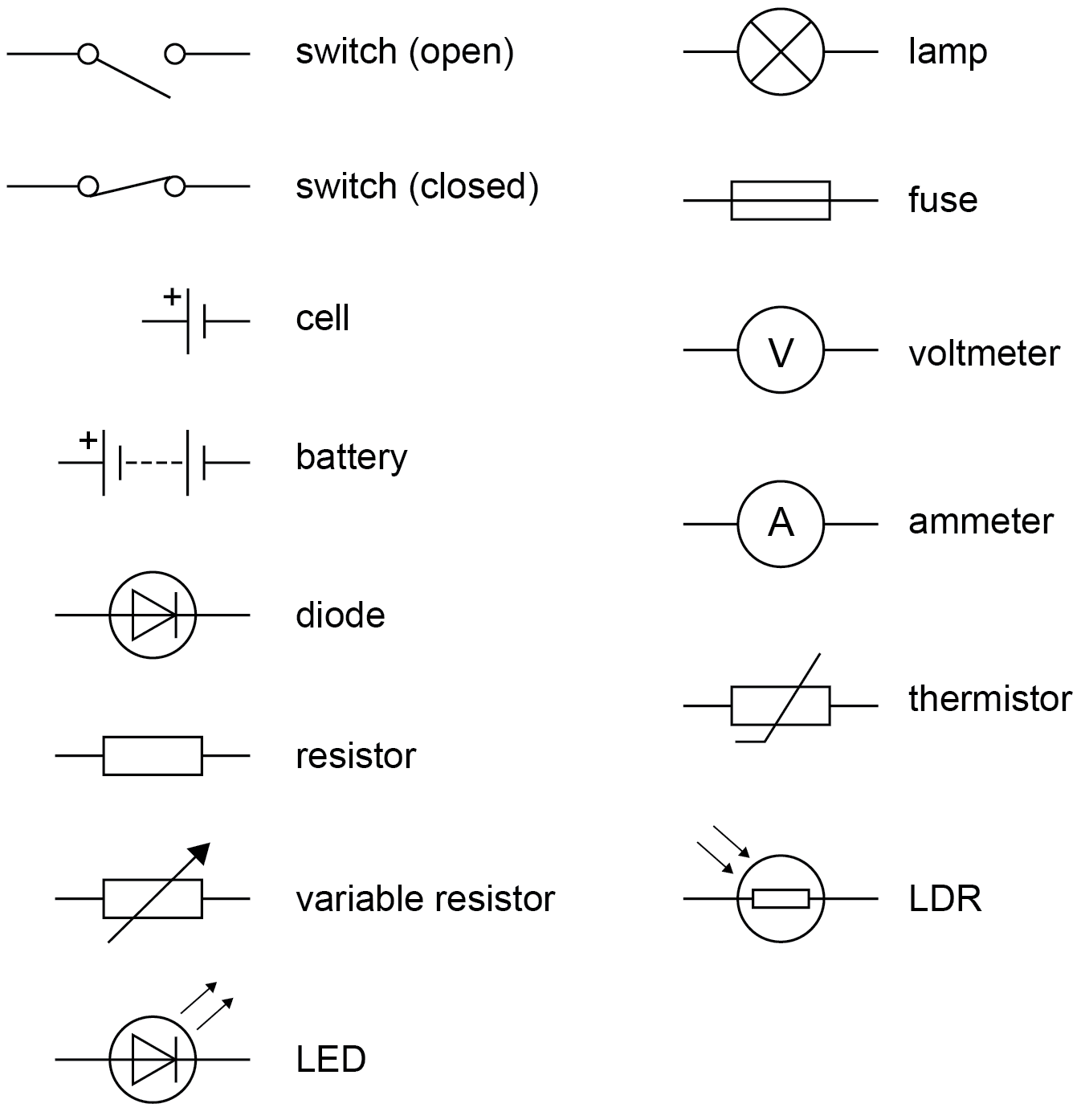 | |
4.7.2.5 Direct and alternating currents
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that the domestic supply in the UK is ac, at 50Hz and about 230 volts; explain the difference between direct and alternating voltage. | Cells and batteries supply current that always passes in the same direction. This is called direct current (dc). An alternating current (ac) is one that changes direction. In the UK ac supply the current changes direction 50 times per second. | |
4.7.2.6 Mains cables
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall the differences in function between the live, neutral and earth mains wires, and the potential differences between these wires; hence explain that a live wire may be dangerous even when a switch in a mains circuit is open, and explain the dangers of providing any connection between the live wire and earth. | Most electrical appliances are connected to the mains using three-core cable. The insulation covering each wire is colour coded for easy identification. - Live wire – brown
- Neutral wire – blue
- Earth wire – green and yellow stripes
The live wire carries the alternating potential difference from the supply. The neutral wire completes the circuit. The earth wire is a safety wire to stop the appliance becoming live. The potential difference between the live wire and earth (0 V) is about 230 V. The neutral wire is at or close to earth potential (0 V). The earth wire is at 0 V; it carries a current only if there is a fault. Our bodies are at earth potential (0 V). Touching the live wire produces a large potential difference across our body. This causes a current to flow through our body, resulting in an electric shock. | WS 1.5 Identify an electrical hazard in a given context. |
4.7.2.7 Power
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain, with reference to examples, the definition of power as the rate at which energy is transferred. | Power is defined as the rate at which energy is transferred or the rate at which work is done. power, P , in watts, W energy transferred, E , in joules, J time, t , in seconds, s work done, W , in joules, J An energy transfer of 1 joule per second is equal to a power of 1 watt. | WS 1.2, 3.3,MS 3b, 3c Recall and apply both of these equations. |
Explain how the power transfer in any circuit device is related to the potential difference across it and the current, and to the energy changes over a given time. | The power of an electrical device is related to the potential difference across it and the current through it by the equation: power, P , in watts, W potential difference, V , in volts, V current, I , in amperes, A (amp is acceptable for ampere) resistance, R , in ohms, Ω | WS 1.2, 3.3, MS 3b, 3c Recall and apply both of these equations. |
4.7.2.8 Power and domestic electric appliances
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe how, in different domestic devices, energy is transferred from batteries and the ac mains to the energy of motors or of heating devices. Describe all the changes involved in the way energy is stored when a system changes, for common situations: bringing water to a boil in an electric kettle. Describe, with examples, the relationship between the power ratings for domestic electrical appliances and the changes in stored energy when they are in use. Describe and calculate the changes in energy involved when a system is changed by work done when a current flows. | Everyday electrical appliances are designed to bring about energy transfers. The amount of energy an appliance transfers depends on how long the appliance is switched on for and the power of the appliance. Work is done when charge flows in a circuit. The amount of energy transferred can be calculated using the equations: energy transferred, E , in joules, J power, P , in watts, W time, t , in seconds, s charge flow, Q , in coulombs, C potential difference, V , in volts, V | WS 1.4 Explain everyday and technological applications of science. WS 1.2, 3.3, MS 3c Recall and apply both of these equations. |
4.7.2.9 The National Grid
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
| Recall that, in the national grid, electrical power is transferred at high voltages from power stations and then transferred at lower voltages in each locality for domestic use; and explain how this system is an efficient way to transfer energy. | The National Grid is a system of cables and transformers linking power stations to consumers. Electrical power is transferred from power stations to consumers using the National Grid. Step-up transformers are used to increase the potential difference from the power station to the transmission cables then step-down transformers are used to decrease the potential difference to a much lower value for domestic use. (HT only) Students should be able to select and use the equation: potential difference across primary coil x current in primary coil = potential difference across secondary coil x current in secondary coil as given on the equation sheet. | WS 1.4 Explain everyday and technological applications of science. Detailed knowledge of the structure of a transformer is not required. |
4.7.3 Acids and alkalis
Some chemical substances can be classified as acids or alkalis. Characterising substances in this way helps to make sense of how chemicals react together, to establish patterns and to make predictions about chemical changes. This topic provides opportunities to write chemical formulae and equations, and apply the quantitative methods from Chemical quantities . Practical content includes methods used to prepare and purify soluble salts. Theoretical content includes how to describe the energy changes associated with neutralisation reactions and how ionic theory can account for the similarities in the reactions of acids.
There are two required practicals: one is the preparation of a pure, dry sample of a soluble salt from an insoluble substance. The other investigates the variables that affect temperature changes in solutions.
4.7.3.1 Reactions of acids
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that acids react with some metals and with carbonates, and write equations predicting products from given reactants. Describe tests to identify selected gases including hydrogen and carbon dioxide. | Acids react with some metals to produce salts and hydrogen. Knowledge of reactions with metals is limited to those of magnesium, zinc and iron with hydrochloric and sulfuric acids. The test for hydrogen uses a burning splint held at the open end of a test tube of the gas. Acids react with metal carbonates to produce salts, water and carbon dioxide. The test for carbon dioxide uses an aqueous solution of calcium hydroxide (limewater). | WS 1.2 (HT only) Explain, in terms of gain or loss of electrons, that the reactions of metals with acids are redox reactions (see Atoms into ions and ions into atoms ). WS 4.1 (HT only) Identify which species are oxidised and which are reduced in given chemical reactions. |
4.7.3.2 Making salts
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe neutralisation as acid reacting with alkali to form a salt plus water. Describe, explain and exemplify the processes of filtration and crystallisation. Suggest suitable purification techniques given information about the substances involved. | Acids are neutralised by alkalis (eg soluble metal hydroxides) and bases (eg insoluble metal hydroxides and metal oxides) to produce salts and water, and by metal carbonates to produce salts, water and carbon dioxide. The particular salt produced in any reaction between an acid and a base or alkali depends on: - the acid used (hydrochloric acid produces chlorides, nitric acid produces nitrates and sulfuric acid produces sulfates)
- the positive ions in the base, alkali or carbonate.
Soluble salts can be made from acids by reacting them with solid insoluble substances such as metals, metal oxides, hydroxides or carbonates. The solid is added to the acid until no more reacts, and the excess solid is filtered off to produce a solution of the salt. Salt solutions can be crystallised to produce solid salts. | WS 1.2 Predict products from given reactants. Use the formulae of common ions to deduce the formulae of salts. |
Required practical activity 17: preparation of a pure, dry sample of a soluble salt from an insoluble oxide or carbonate, using a Bunsen burner and a water bath or electric heater to evaporate the solution.
AT skills covered by this practical activity: chemistry AT 2, 3, 4 and 6.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.3.3 Energy changes and reactions
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Distinguish between endothermic and exothermic reactions on the basis of the temperature change of the surroundings. | When chemical reactions occur, energy is transferred to or from the surroundings. Energy is conserved in chemical reactions. The total amount of energy in the reaction mixture and its surroundings at the end of a chemical reaction is the same as it was at the start. An exothermic reaction is one that gives out energy. This heats up the reaction mixture. Energy then transfers to the surroundings as the reaction mixture then cools. Neutralisation of an acid with an alkali is an example of an exothermic reaction. An endothermic reaction is one that that takes in energy. This cools the reaction mixture. Energy then transfers from the surroundings as the reaction mixture then warms up again. The reaction of citric acid and sodium hydrogen carbonate is an example of an endothermic reaction. | WS 1.2 Identify examples of exothermic and endothermic reactions based on the temperature change of the reaction mixture. |
Required practical activity 18: investigate the variables that affect the temperature changes of a series of reactions in solutions, eg acid plus metals, acid plus carbonates, neutralisations, displacement of metals.
AT skills covered by this practical activity: chemistry AT 1, 3, 5 and 6.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.3.4 The pH scale and neutralisation
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions. (HT only) Use the formulae of common ions to write balanced ionic equations. Recall that relative acidity and alkalinity are measured by pH. Recognise that aqueous neutralisation reactions can be generalised to hydrogen ions reacting with hydroxide ions to form water. | Acids produce hydrogen ions (H+ ) in aqueous solutions. Aqueous solutions of alkalis contain hydroxide ions (OH– ). The pH scale, from 0 to 14, is a measure of the acidity or alkalinity of a solution and can be measured using universal indicator or a pH probe. A solution with pH 7 is neutral. Aqueous solutions of acids have pH values of less than 7 and aqueous solutions of alkalis have pH values greater than 7. In neutralisation reactions between an acid and an alkali, hydrogen ions react with hydroxide ions to produce water. | WS 1.2 Write an ionic equation to represent neutralisation. WS 2.3 Describe the use of universal indicator or a wide range indicator to measure the approximate pH of a solution. WS 3.2 Use the pH scale to identify acidic or alkaline solutions. |
4.7.3.5 Strong and weak acids (HT only)
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Use and explain the terms dilute and concentrated (amount of substance) and weak and strong (degree of ionisation) in relation to acids. Recall that as hydrogen ion concentration increases by a factor of ten the pH value of a solution decreases by a factor of one. Describe neutrality and relative acidity and alkalinity in terms of the effect of the concentration of hydrogen ions on the numerical value of pH (whole numbers only). | A strong acid is completely ionised in aqueous solution. Examples of strong acids are hydrochloric, nitric and sulfuric acids. A weak acid is only partially ionised in aqueous solution. Examples of weak acids are ethanoic, citric and carbonic acids. For a given concentration of aqueous solutions, the stronger an acid, the lower the pH. | |
4.7.4 The rate and extent of chemical change
Chemical reactions can occur at very different rates. Although the reactivity of chemicals is a significant factor in how fast chemical reactions proceed, there are many variables that can be manipulated in order to speed them up or slow them down. Catalysts, including enzymes, can have a very significant effect on reaction rates. An understanding of the energy changes that accompany bond breaking and bond forming can help to account for the effect of temperature on rates. Chemical reactions may also be reversible and therefore the effect of different variables needs to be established in order to identify how to maximise the yield of desired product.
There are two required practicals: an investigation of the effect of changes in concentration on the rate of a chemical reaction and an investigation of a factor affecting the rate of an enzyme-controlled reaction.
4.7.4.1 Factors that affect reaction rates
GCSE Science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe the effect of changes in temperature, concentration, pressure, and surface area on rate of reaction. Suggest practical methods for determining the rate of a given reaction. | The rate of a chemical reaction can be found by measuring the quantity of a reactant used or the quantity of product formed over time: The quantity of reactant or product can be measured by the mass in grams, by a volume in cm3 ((HT only) or by an amount in moles). The units of rate of reaction may be given as g/s, cm3 /s ((HT only) or mol/s). The rate of a chemical reaction can be determined by measuring: - the loss in mass of a reactant's mixture
- the volume of gas produced
- the time for a solution to become opaque or coloured.
| WS 3.3, MS 1a, 1c Calculate the mean rate of a reaction from given information about the quantity of a reactant used or the quantity of a product formed and the time taken. WS 3.5, MS 4a, 4b, 4c Draw, and interpret, graphs showing the quantity of product formed or quantity of reactant used up against time to compare or determine rates of reaction. WS 3.3, MS 4e Draw tangents to the curves on these graphs and use the gradient of the tangent as a measure of the rate of reaction. WS 3.3, MS 4d, 4e (HT only) Calculate the gradient of a tangent to the curve on these graphs as a measure of rate of reaction at a specific time. |
4.7.4.2 The effect of surface area on rates of reaction
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain the effects on rates of reaction of changes in the size of the pieces of a reacting solid in terms of surface area to volume ratio. | Breaking up a solid reactant into smaller pieces increases the surface area that can be in contact with any solution with which it reacts. Increasing the ratio of surface area to volume increases the rate of reaction for a given mass of a solid reactant. | MS 1c Use proportionality when comparing factors affecting rate of reaction. MS 5c Calculate surface areas and volumes of cubes. |
4.7.4.3 The effect of temperature, concentration and pressure on rates of reaction
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain the effects on rates of reaction of changes in temperature, concentration and pressure in terms of the frequency and energy of collision between particles. | According to collision theory, chemical reactions can occur only when reacting particles collide with each other and with sufficient energy. The minimum amount of energy that particles must have to react is called the activation energy. Increasing the concentration of reactants in solution, the pressure of reacting gases and the surface area of solid reactants increases the frequency of collisions and so increases the rate of reaction. Increasing the temperature increases the frequency of collisions and makes the collisions more energetic, and so increases the rate of reaction. | WS 1.2 Predict and explain the effects of changing conditions on the rate of a reaction. |
Required practical activity 19: investigation of how changes in concentration affect the rates of reactions by a method involving measuring the volume of a gas produced and a method involving a change in colour or turbidity. This should be an investigation involving developing a hypothesis.
AT skills covered by this practical activity: chemistry AT 1, 3, 5 and 6.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.4.4 Activation energy
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain activation energy as the energy needed for a reaction to occur. Draw and label a reaction profile for an exothermic and an endothermic reaction, identifying activation energy. | Chemical reactions can occur only when reacting particles collide with each other and with sufficient energy. The minimum amount of energy that particles must have to react is called the activation energy. Reaction profiles can be used to show the relative energies of reactants and products, the activation energy and the overall energy change of a reaction. | WS 3.2, 3.5, MS 4a Interpret reaction profiles, including using them to identify reactions as exothermic or endothermic. |
4.7.4.5 Bond breaking and bond forming (HT only)
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
| Calculate energy changes in a chemical reaction by considering bond making and bond breaking energies. | During a chemical reaction: - energy must be supplied to break bonds in the reactants
- energy is given out when bonds in the products are formed.
The energy needed to break bonds and the energy given out when bonds are formed can be calculated from bond energies. The difference between the sum of the energy needed to break bonds in the reactants and the sum of the energy given out when bonds in the products are formed is the overall energy change of the reaction. In an exothermic reaction, the energy given out from forming new bonds is greater than the energy needed to break existing bonds. In an endothermic reaction, the energy needed to break existing bonds is greater than the energy given out from forming new bonds. | WS 3.3, MS 1a Use arithmetic computation when calculating energy changes. WS 1.2, 3.3, MS 1a, 4a Calculate the energy transferred in chemical reactions between simple molecules in the gas state using bond energies supplied. |
4.7.4.6 Catalysts
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe the characteristics of catalysts and their effect on rates of reaction. Identify catalysts in reactions. Explain catalytic action in terms of activation energy. | Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Knowledge of the names of catalysts other than those specified in the subject content is not required. | WS 3.5 Identify catalysts in reactions from their effect on the rate of reaction and because they are not included in the chemical equation for the reaction. WS 1.2 Use reaction profiles to explain catalytic action. |
4.7.4.7 Enzymes
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that enzymes act as catalysts in biological systems. Explain the mechanism of enzyme action including the active site, enzyme specificity and factors affecting the rate of enzymatic reaction. | Enzymes are important as biological catalysts which allow all the reactions in cells to occur. Enzymes are large protein molecules. The shape of an enzyme is vital for its function. Each enzyme has an active site with a unique shape to bind a specific substrate molecule. High temperatures and extremes of pH denature the enzyme, changing the shape of the active site. The 'lock and key' model is a simplified model of enzyme action. Different enzymes work fastest at different temperatures and pH values. | WS 3.3, 3.5, MS 1a, 1c, 1d Carry out rate calculations for chemical reactions and make estimates of simple calculations without using a calculator. |
Required practical activity 20: investigate the effect of pH on the rate of reaction of amylase enzyme. Students should use a continuous sampling technique to determine the time taken to completely digest a starch solution at a range of pH values. Iodine reagent is to be used to test for starch every 30 seconds. Temperature must be controlled by use of a water bath or electric heater.
AT skills covered by this practical activity: biology AT 1, 2 and 5.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.4.8 Reversible reactions
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that some reactions may be reversed by altering the reaction conditions. | In some chemical reactions, the products of the reaction can react to produce the original reactants. Such reactions are called reversible reactions and are represented: The direction of reversible reactions can be changed by changing the temperature. Examples include the effect of changing the temperature on the decomposition of ammonium chloride and of hydrated copper(II) sulfate. | |
4.7.4.9 Dynamic equilibrium
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Recall that dynamic equilibrium occurs when the rates of forward and reverse reactions are equal. | When a reversible reaction occurs in apparatus which prevents the escape of reactants and products, equilibrium is reached when the forward and reverse reactions occur at exactly the same rate. | |
4.7.4.10 Factors affecting the position of equilibrium (HT only)
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Predict the effect of changing reaction conditions on equilibrium position. | The relative amounts of all the reactants and products at equilibrium depend on the conditions of the reaction. If a system is at equilibrium and a change is made to any of the conditions, then the system responds to counteract the change. | WS 1.2 Apply Le Châtelier's principle to make qualitative predictions about the effect of changes on systems at equilibrium when given appropriate information. |
Predict the effect of changing concentration on equilibrium position and suggest appropriate conditions to produce a particular product. | If the concentration of one of the reactants or products is changed, the system is no longer at equilibrium and the concentrations of all the substances change until equilibrium is reached again. If the concentration of a reactant is increased, more products form until equilibrium is reached again. If the concentration of a product is decreased, more reactants react until equilibrium is reached again. | WS 3.5 Interpret appropriate given data to predict the effect of a change in concentration of a reactant or product on given reactions at equilibrium. |
Predict the effect of changing temperature on equilibrium position and suggest appropriate conditions to produce a particular product. | If the temperature of a system at equilibrium is increased: - the relative amount of products at equilibrium increases for an endothermic reaction
- the relative amount of products at equilibrium decreases for an exothermic reaction.
If the temperature of a system at equilibrium is decreased: - the relative amount of products at equilibrium decreases for an endothermic reaction
- the relative amount of products at equilibrium increases for an exothermic reaction.
| WS 1.2 Apply the idea that if a reversible reaction is exothermic in one direction, it is endothermic in the opposite direction. |
Predict the effect of changing pressure on equilibrium position and suggest appropriate conditions to produce a particular product. | For gaseous reactions at equilibrium: - an increase in pressure causes the equilibrium position to shift towards the side with the smaller number of molecules, as shown by the symbol equation for that reaction
- a decrease in pressure causes the equilibrium position to shift towards the side with the larger number of molecules, as shown by the symbol equation for that reaction.
| |
4.7.5 Atoms into ions and ions into atoms
Metals can be arranged in an activity series in terms of the ability of their atoms to turn into positive ions. Electrolysis is a process that reverses such changes by turning ions back into atoms (and sometimes molecules). Gas tests are introduced to identify the products of electrolysis. Gain or loss of electrons can be used to classify electrode reactions as reduction or oxidation processes.
The required practical is an investigation of the electrolysis of aqueous solutions using inert electrodes.
4.7.5.1 A reactivity series for metals
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain how the reactivity of metals with water or dilute acids is related to the tendency of the metal to form its positive ion. | When metals react with other substances the metal atoms form positive ions. Metals can be arranged in order of their reactivity in a reactivity series. The metals potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper can be put in order of their reactivity from their reactions with water and dilute acids. The non-metals hydrogen and carbon are often included in the reactivity series. A more reactive metal can displace a less reactive metal from a compound. The reactions of metals with water and acids are limited to room temperature and do not include reactions with steam. | WS 3.8 Recall and describe the reactions, if any, of potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper with water or dilute acids. WS 3.5 Deduce an order of reactivity of metals based on experimental results. WS 1.2 (HT only) Write ionic equations for displacement reactions. |
4.7.5.2 Electrolysis
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe electrolysis in terms of the ions present and reactions at the electrodes. Recall that metals (or hydrogen) are formed at the cathode and non-metals are formed at the anode in electrolysis using inert electrodes. | When an ionic compound is melted or dissolved in water, the ions are free to move about within the liquid or solution. These liquids and solutions are able to conduct electricity and are called electrolytes. Passing an electric current through electrolytes causes the ions to move to the electrodes. Positively charged ions move to the negative electrode (the cathode), and negatively charged ions move to the positive electrode (the anode). Ions are discharged at the electrodes producing elements. This process is called electrolysis. When a simple ionic compound is electrolysed in the molten state using inert electrodes, the metal is produced at the cathode and the non-metal is produced at the anode. | WS 1.2 Predict the products of electrolysis of binary ionic compounds in the molten state. (HT only) Write half equations for the reactions occurring at the electrodes during electrolysis. Students may be required to complete and balance supplied half equations. |
4.7.5.3 Electrolysis of aqueous solutions
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe competing reactions in the electrolysis of aqueous solutions of ionic compounds in terms of the different species present. | The ions discharged when an aqueous solution is electrolysed using inert electrodes depend on the relative reactivity of the elements involved. At the negative electrode (cathode), hydrogen is produced if the metal is more reactive than hydrogen. At the positive electrode (anode), oxygen is produced unless the solution contains halide ions when the halogen is produced. This happens because in the aqueous solution water molecules break down producing hydrogen ions and hydroxide ions that are discharged. | WS 1.2 (HT only) Write half equations for the reactions occurring at the electrodes during electrolysis. Students may be required to complete and balance supplied half equations. |
Required practical activity 21: investigate what happens when aqueous solutions are electrolysed using inert electrodes. This should be an investigation involving developing a hypothesis.
AT skills covered by this practical activity: chemistry AT 3 and 7.
This practical activity also provides opportunities to develop WS and MS. Details of all skills are given in Key opportunities for skills development .
4.7.5.4 Tests for gases
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Describe tests to identify selected gases including oxygen, hydrogen and chlorine. | The test for hydrogen uses a burning splint held at the open end of a test tube of the gas. The test for oxygen uses a glowing splint inserted into a test tube of the gas. The test for chlorine uses damp litmus paper put into chlorine gas. | WS 3.5 Interpret the observations from gas tests. |
4.7.5.5 Electron transfer reactions (HT only)
GCSE science subject content | Details of the science content | Scientific, practical and mathematical skills |
|---|
Explain reduction and oxidation in terms of gain or loss of electrons, identifying which species are oxidised and which are reduced. | Oxidation is the loss of electrons and reduction is the gain of electrons. During electrolysis, at the cathode (negative electrode), positively charged ions gain electrons and so the reactions are reductions. At the anode (positive electrode), negatively charged ions lose electrons and so the reactions are oxidations. | WS 4.1 Identify, in a given reaction, symbol equation or half equation, which species are oxidised and which are reduced. |